Related Vendors
The reading is proportional to the combined effect of all ions in the sample and it gives a quick overview of the total dissolved solids in the water. Therefore, it is an important tool for monitoring and surveillance of a wide range of different types of waters (pure water, drinking water, process water, etc.,) and beverages. The higher the content of dissolved solids, the higher is the conductivity. Ultra-pure water has a conductivity of 0.055 μS/cm due to the self-ionization of water. Sea water containing about 35 g salt per liter reaches 55 mS/cm.
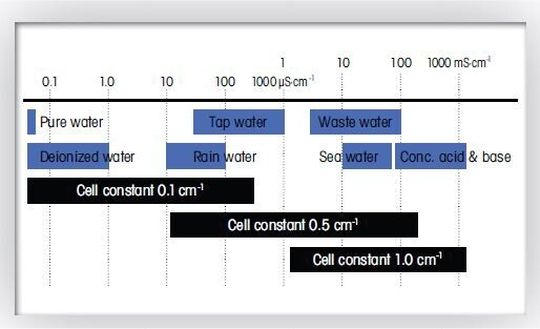
The determination of the electric conductivity requires a conductivity meter and a conductivity cell. It is fast, simple and reliable. One of the most important parameters for the selection of the conductivity cell is the cell constant. Figure 1 shows the recommended cell constant for different conductivity ranges. Whether the user opts for an epoxy, steel or glass shaft depends on the practical conditions of the conductivity measurement. Manufacturers also offer several conductivity meter models providing different levels of performance and operational excellence.
Using Water Analysis for Drinks and Beverages
The methods of the classical water analysis include hardness (calcium, magnesium), p- and m-value, chlorides, fluorides, sulfates, sodium and potassium. The m-value is also called alkalinity, carbonate hardness or temporary hardness. The p-value is likewise referred to phenolphthalein acidity.
Measuring Acidity Correctly
Titration has been applied for centuries to determine the content of acids in various samples. Before the first electrode was invented, color indicators have been used to indicate the endpoint of the titration. Acidity in particular represents a classical parameter in quality control and routine analysis of water and nonalcoholic beverages.
:quality(80)/images.vogel.de/vogelonline/bdb/772600/772665/original.jpg)
Arise in the Midst of Challenges
Increasing opportunities for the food and beverage sector
Fruit juices contain organic acids such as citric, tartatric, lactic, malic, ascorbic or acetic. Carbonated soft drinks may contain inorganic phosphoric acid. These acids contribute to the acidity of the final product. Generally, the titration of acids in nonalcoholic beverages and water is rather straightforward: The acid content is determined by controlled addition of an alkaline titrant solution of known concentration (e.g., sodium hydroxide) until a specific endpoint is reached.
Details of the acid content determination are regulated by various national or international entities including standard owners such as the Association of Official Analytical Chemists (AOAC, USA), the Organisation International de la Vigne et du Vin (OIV, FR), the International Organization for Standardization (ISO) and regulatory bodies like the Food and Drug Administration (FDA, UA).
(ID:43164568)



:quality(80)/p7i.vogel.de/wcms/f3/4a/f34abcf99d71d8bff5a4e98b60889c8e/0129239597v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/5c/27/5c27bddaef122b61c636e31d82a33bd9/0129017834v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/f7/f0/f7f0fdf4a4e1d872d078d63abfa4a7fd/0127844838v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/1e/50/1e5099910c2370ad08d578161f3fe3c2/0128939735v1.jpeg)
:quality(80)/p7i.vogel.de/wcms/ed/af/edaf3b0b2506f65f338232550b661eb3/0129281129v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/66/29/66293f3c9de08b472f095dc9602bcc7d/0129279548v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/5b/e1/5be15b3ec731f81589ae9b16a43ac54c/0129243604v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/40/6d/406de78846e840f6c7cf7849cbf0ab08/0129163370v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/2c/87/2c879e9ba1bd966c7003802f0b8e1149/0128880738v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/f2/d4/f2d42a2b7ad97329aeafb49c514f63f3/0128879848v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/94/f2/94f2a278fd90b0da7400966874f44950/0128605000v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/1f/52/1f528d0628ffe679a69a2e9f65c0e157/0128668896v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/d0/1b/d01bf797fa89982391d0ac726c3d69aa/0129278531v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/03/11/031137c1a0c2b4407960a762fd0fd915/0129189201v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/47/4f/474fac12fca989a8d8db63de335dd78d/0129188583v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/cd/1f/cd1f2c8c755b62b5693a7a25ceb830e8/0129165850v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/be/e1/bee13b8d51a419b9b8f0685e2b9d47bf/0128917673v1.jpeg)
:quality(80)/p7i.vogel.de/wcms/51/7a/517a4e83c5827663b4839f5ddb98f434/0128872953v1.jpeg)
:quality(80)/p7i.vogel.de/wcms/d5/72/d5728578b35b72f365f5086bc1d068da/0128662441v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/a9/69/a96920e47e362a9cf0b9916b7ad3d30a/0128071795v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/18/47/1847e6b1456c96f04bd511bbd21f3779/0129039479v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/3b/fd/3bfda4bbcb1a60004c330bde3b705109/0128453300v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/cc/19/cc19aa5fec6f6f2b25dbd9516efed735/0128077403v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/de/66/de66dd32e1af2fa37ff20bded4417afc/0127787418v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/6b/cc/6bcc3d26e1c7c74d90a10bc3ca296ae1/0128362351v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/24/c8/24c84ab6b1bcd24468a87820a07a85aa/0128194707v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/1d/3e/1d3e2788eb37c1e974f36337c8d33cb5/0128191010v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/19/76/1976928d7d0a2ed7c1eeba7ade8552c8/0126365603v1.jpeg)
:quality(80)/p7i.vogel.de/wcms/d1/a8/d1a83b822e16733380268322d033abdc/0129239584v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/02/02/020250273eb250f46d355a16c1baeb74/0129163933v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/71/3b/713bcd0d20244ceda895e06bd851d5a9/0129162836v3.jpeg)
:quality(80)/p7i.vogel.de/wcms/41/22/412299226dfb0b70c11970e2f3f339e3/0129131672v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/f8/29/f8298429c1638b949a6f76346f6709c7/0118701710v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/07/24/07242664ab2b1c7841c9d9d0a127670c/0116045959.jpeg)
:quality(80)/p7i.vogel.de/wcms/c9/79/c979a20b32395ddfa93fe7ead90578a0/0108386061.jpeg)
:quality(80)/p7i.vogel.de/wcms/9e/5c/9e5c92d942ed046a27562d6e3d730c92/0103483548.jpeg)
:quality(80)/p7i.vogel.de/wcms/16/3d/163da381529db3a47348a9440655529b/0125732969v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/e7/82/e782bbbf96e4971c22241d76e5de1720/0124855387v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/f9/40/f940b2630f4805f08212fd851af46d6d/0124788233v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/3a/6d/3a6d046cf1db266c3009cecb2af8e2b7/0124656182v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/a8/4e/a84e8039a90a5cf4751d01ebcf6ba1a9/0127510172v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/17/c7/17c703445f134eb3d7ecc7918dda2762/0124596096v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/2a/2c/2a2cffc07f51019065387cd63241b5ce/0119463370v1.jpeg)
:quality(80)/p7i.vogel.de/wcms/b1/7e/b17ea8c62ccafad1d1fb072d6199bbd6/0118578446.jpeg)
:quality(80)/p7i.vogel.de/wcms/bb/54/bb54162539c6fc9ac135719af078ea06/0129188218v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/3f/69/3f69ed74aeaa46ac08dcd9fbe1f2d497/0129141343v2.jpeg)
:fill(fff,0)/images.vogel.de/vogelonline/companyimg/107800/107832/65.jpg)
:fill(fff,0)/p7i.vogel.de/companies/68/07/6807ad98568ce/logo-elementar-rgb.jpeg)
:fill(fff,0)/p7i.vogel.de/companies/68/c8/68c815bc8fe81/prominent-logo-300x300.jpeg)
:quality(80)/p7i.vogel.de/wcms/27/6f/276f0df09fd968e74c7c58d5915d07f1/0127144840v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/c1/ed/c1ed89188a8fdb351601e36204d4c38b/0125059797v2.jpeg)