Related Vendors
Currently, gel packs (water with a viscosity modifier to convert it into a gel form for ease of handling) are packed inside insulated boxes made of expanded polystyrene (EPS), polyurethane foam (PU) or more recently vacuum insulated panels (VIP). The gels packs, packed in pouches, are frozen in a deep freezer and act as a cooling agent for vaccines.
It is essentially ice. This offers a cheap solution as compared to an active container that run on battery or is powered by fuel. However, it has been observed that gel packs are not very successful and vaccines packed with them ceases to be effective.
A Losing Strategy for Big Pharma?
For refrigerated products, that have to be maintained between 2°C to 8°C, gel packs prove to be inoperative as they freeze at temperatures much below 0°C. When vaccines come in contact with these packs, the temperatures go below 2°C. This results in a temperature shock and vaccine lose its potency. Due to lack of stringent government standards, freight forwarders continue using them as it can provide a cheaper solution.
For frozen products, conventionally, dry ice is used to maintain subzero temperature. This leads to temperatures going as low as -60°C to -80°C, which is unacceptable for the vaccines. Pharmaceutical companies must understand that such solutions end up into a losing strategy and loss of effort and millions spent by international bodies like WHO, UNICEF, World Bank and private philanthropic foundations
Why a Switch from Dry Ice is Needed
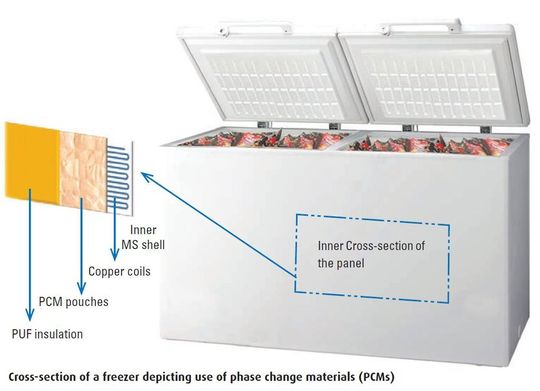
The solutions are twofold. First is the right technology. A switch from dry ice, gel packs and water is the need of the hour. Newer materials, such as phase change materials (PCMs), can provide an efficient alternative to eliminate the threats posed by existing choices of pharma transport. These are specially designed mixtures of chemicals that can absorb large amount of heat without a significant rise in temperature. This is known as the latent heat.
:quality(80)/images.vogel.de/vogelonline/bdb/440800/440890/original.jpg)
Single–Use Bioreactors
Single–Use Reactors: A Panacea for Future
Unlike gel packs which freeze at below 0°C, PCMs can be specially designed to cater to a specific temperature. For instance, to maintain a vaccine between a temperature range of 2–8°C, a specially designed PCM to change phase at 5°C could eventually store the vaccine well within the efficacy standards.
(ID:43040921)



:quality(80)/p7i.vogel.de/wcms/f7/f0/f7f0fdf4a4e1d872d078d63abfa4a7fd/0127844838v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/1e/50/1e5099910c2370ad08d578161f3fe3c2/0128939735v1.jpeg)
:quality(80)/p7i.vogel.de/wcms/56/ec/56ecda05b645f0174c02631d95ca9aa4/0128668888v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/e2/dc/e2dc8cc128d8291e54f37ca35e8248e5/0128664847v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/e7/40/e74003abab999d9c5ff06672ed899136/0128971984v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/0b/c2/0bc2d35b3728c6155bcdc18aa0b4f43e/0128940631v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/ad/82/ad8217d5688276d9ed82b41be17e2edb/0128939746v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/fa/04/fa0408f795513d992edc5f098924e404/0128934009v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/2c/87/2c879e9ba1bd966c7003802f0b8e1149/0128880738v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/f2/d4/f2d42a2b7ad97329aeafb49c514f63f3/0128879848v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/94/f2/94f2a278fd90b0da7400966874f44950/0128605000v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/1f/52/1f528d0628ffe679a69a2e9f65c0e157/0128668896v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/cf/5b/cf5b66e0b2268fdadb5e20fd585edebb/0128971089v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/c1/67/c167f473ddeca77220259be63c52e80f/0128933152v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/51/7a/517a4e83c5827663b4839f5ddb98f434/0128872953v1.jpeg)
:quality(80)/p7i.vogel.de/wcms/be/e1/bee13b8d51a419b9b8f0685e2b9d47bf/0128917673v1.jpeg)
:quality(80)/p7i.vogel.de/wcms/d5/72/d5728578b35b72f365f5086bc1d068da/0128662441v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/a9/69/a96920e47e362a9cf0b9916b7ad3d30a/0128071795v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/3b/fd/3bfda4bbcb1a60004c330bde3b705109/0128453300v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/cc/19/cc19aa5fec6f6f2b25dbd9516efed735/0128077403v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/de/66/de66dd32e1af2fa37ff20bded4417afc/0127787418v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/d5/78/d578d9a15e78a46fad46847d963a04c2/0127547393v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/6b/cc/6bcc3d26e1c7c74d90a10bc3ca296ae1/0128362351v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/24/c8/24c84ab6b1bcd24468a87820a07a85aa/0128194707v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/1d/3e/1d3e2788eb37c1e974f36337c8d33cb5/0128191010v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/19/76/1976928d7d0a2ed7c1eeba7ade8552c8/0126365603v1.jpeg)
:quality(80)/p7i.vogel.de/wcms/d4/ba/d4ba61e95ad0cdd0e150bb37d0f93afe/0128971702v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/b1/3e/b13e690924b3d0318ad71289acfd6b82/0128934399v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/89/8e/898eadf3c417034960d4afb39507d332/0128898269v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/c3/36/c336be5ddcc860374b32bd808d0bbd60/0128897614v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/f8/29/f8298429c1638b949a6f76346f6709c7/0118701710v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/07/24/07242664ab2b1c7841c9d9d0a127670c/0116045959.jpeg)
:quality(80)/p7i.vogel.de/wcms/c9/79/c979a20b32395ddfa93fe7ead90578a0/0108386061.jpeg)
:quality(80)/p7i.vogel.de/wcms/9e/5c/9e5c92d942ed046a27562d6e3d730c92/0103483548.jpeg)
:quality(80)/p7i.vogel.de/wcms/16/3d/163da381529db3a47348a9440655529b/0125732969v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/e7/82/e782bbbf96e4971c22241d76e5de1720/0124855387v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/f9/40/f940b2630f4805f08212fd851af46d6d/0124788233v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/3a/6d/3a6d046cf1db266c3009cecb2af8e2b7/0124656182v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/a8/4e/a84e8039a90a5cf4751d01ebcf6ba1a9/0127510172v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/17/c7/17c703445f134eb3d7ecc7918dda2762/0124596096v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/2a/2c/2a2cffc07f51019065387cd63241b5ce/0119463370v1.jpeg)
:quality(80)/p7i.vogel.de/wcms/b1/7e/b17ea8c62ccafad1d1fb072d6199bbd6/0118578446.jpeg)
:quality(80)/p7i.vogel.de/wcms/a3/f6/a3f6cf659bef4a255272c8af711c20af/0128599957v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/d7/b4/d7b4415fef8a0be11c7f8fd4197edc92/0128599865v2.jpeg)
:fill(fff,0)/images.vogel.de/vogelonline/companyimg/107800/107832/65.jpg)
:fill(fff,0)/images.vogel.de/vogelonline/companyimg/2000/2093/65.jpg)
:fill(fff,0)/p7i.vogel.de/companies/61/f9/61f9439b7cbe4/logo-edl.png)
:quality(80)/p7i.vogel.de/wcms/5e/b1/5eb1560763d85ad5d5a286dfedcdeaaf/0124211427v2.jpeg)
:quality(80)/p7i.vogel.de/wcms/c1/ed/c1ed89188a8fdb351601e36204d4c38b/0125059797v2.jpeg)